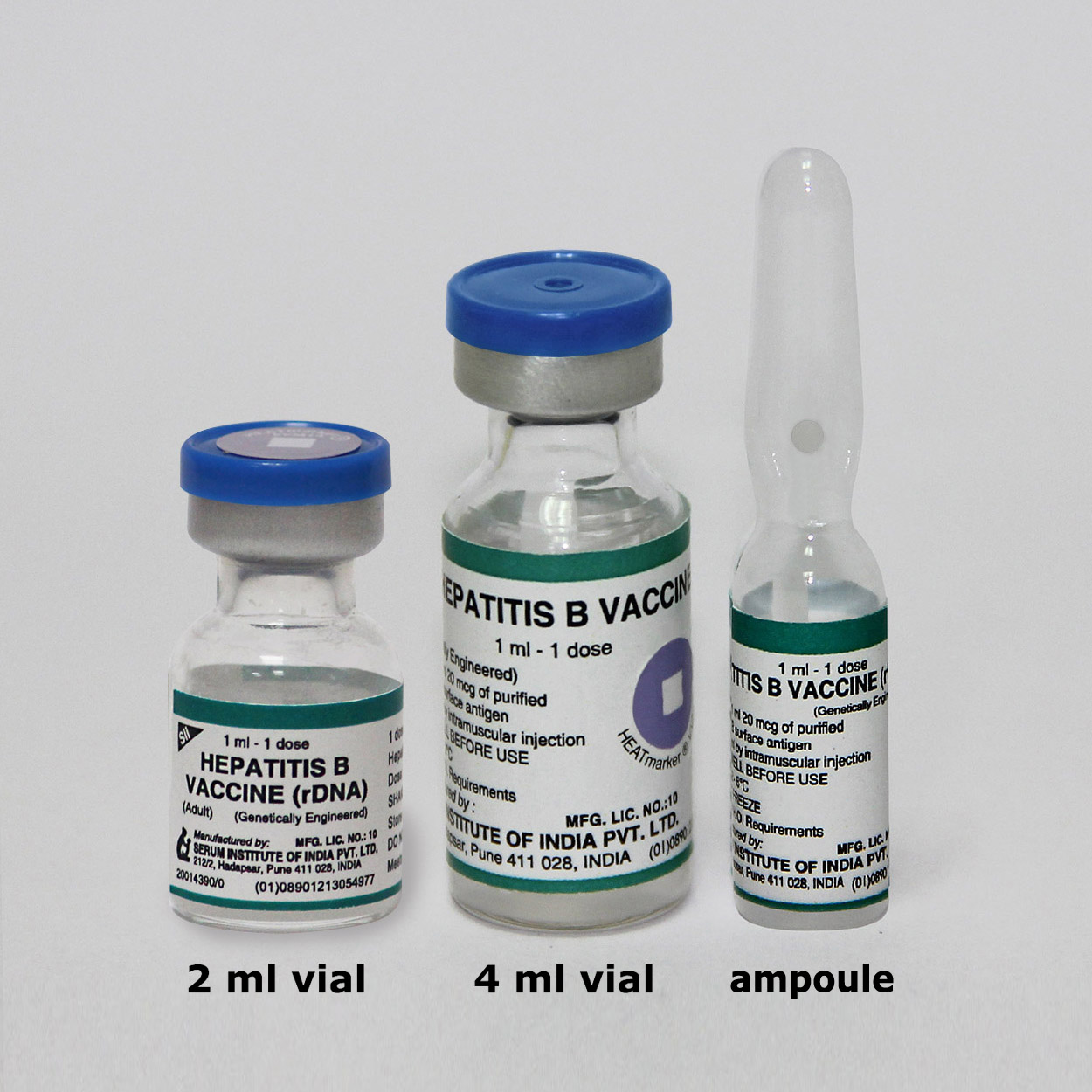
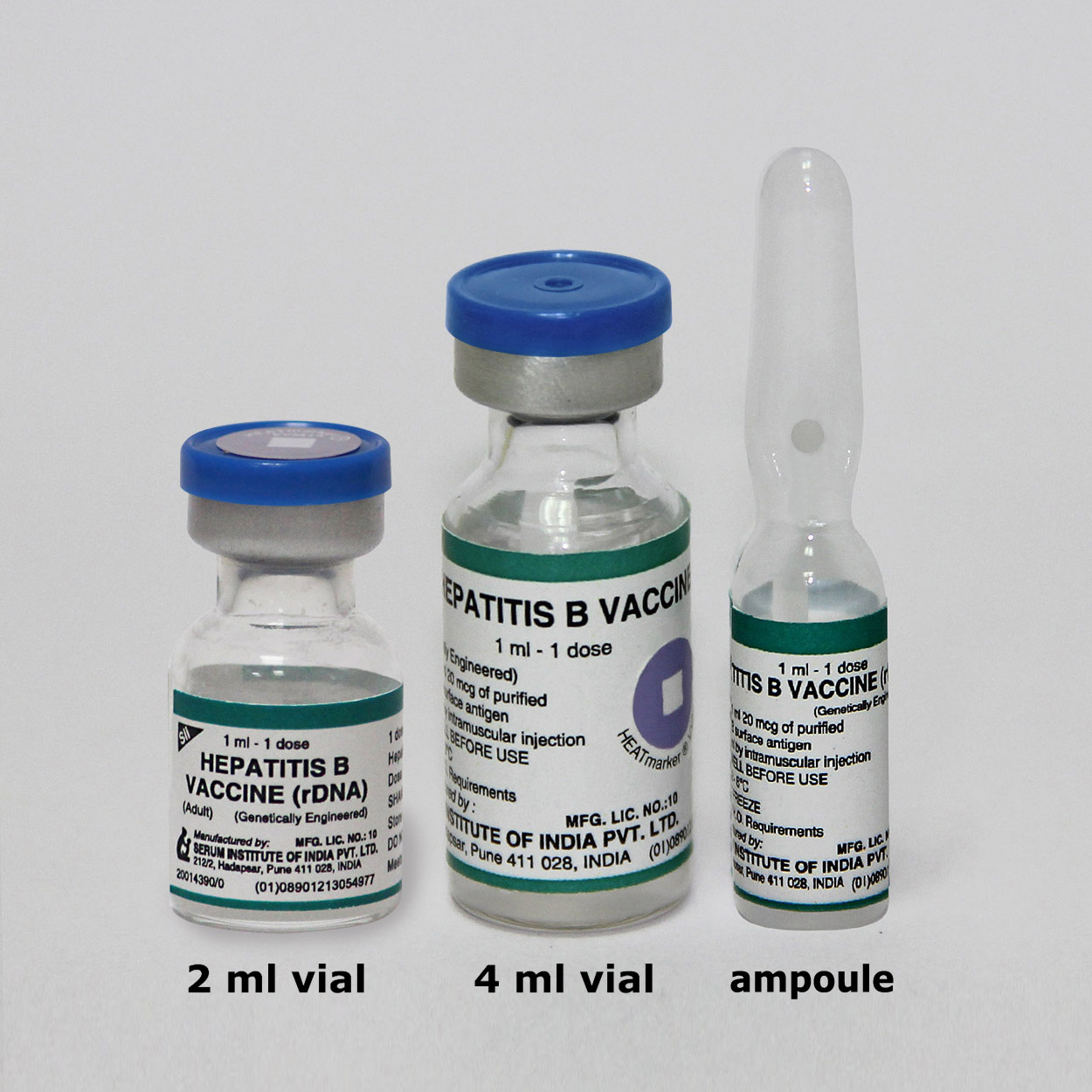
Hepatitis B Vaccine (rDNA) (Adult)
Hepatitis B vaccine (recombinant)
Product Details
WHO Product ID
FVP-P-131
Status
Prequalified
Date of prequalification
Vaccine type
Hepatitis B vaccine (recombinant)
Abbreviated Name
HepB
Commercial Name
Hepatitis B Vaccine (rDNA) (Adult)
PQ Holder
Serum Institute of India Pvt. Ltd.
212/2 Off Soli Poonawala Road, Hadaspar
Pune,
Maharashtra
411 028
India
Responsible NRA
Central Drugs Standard Control Organization (CDSCO)
Product description
Pharmaceutical Form
Liquid: Ready to use
Presentation
Vial
Number of doses
1
Route of administration
Intramuscular
Vaccine Vial Monitor
Type 30
Multidose Vial Policy
Not applicable
Shelf life (months)
36 months
Storage temperature
2 - 8°C
Diluent
N/A
Preservative
Thiomersal
Preservative concentration
0.01%
Remarks
cold chain volume (cc)/dose: 17.575 (vial); 12.18 (ampoule)Ampoule does not hold Vaccine Vial Monitor (VVM)
Product Packaging
Secondary Container
Tertiary Container
Component Packed
Active (Vaccine)
Total doses
50
Description
Carton of 50 vials (50 doses) [Dimensions 18.5 x 9.5 x 5.0 (4 ml)]
Dimensions
18.5 x 9.5 x 5.0 cm
Cold Chain Volume (cm³/dose)
17.58
Component Packed
Active (Vaccine)
Total doses
50
Description
Carton of 50 vials (50 doses)[Dimensions: 18.5 x 9.5 x 4.0 (2 ml)]
Dimensions
18.5 x 9.5 x 4.0 cm
Cold Chain Volume (cm³/dose)
14.06
Component Packed
Active (Vaccine)
Total doses
50
Description
Carton of 50 ampoules (50 doses) [Dimensions: 14.5 x 6.0 x 7.0 cm]
Dimensions
14.5 x 6.0 x 7.0 cm
Cold Chain Volume (cm³/dose)
12.18