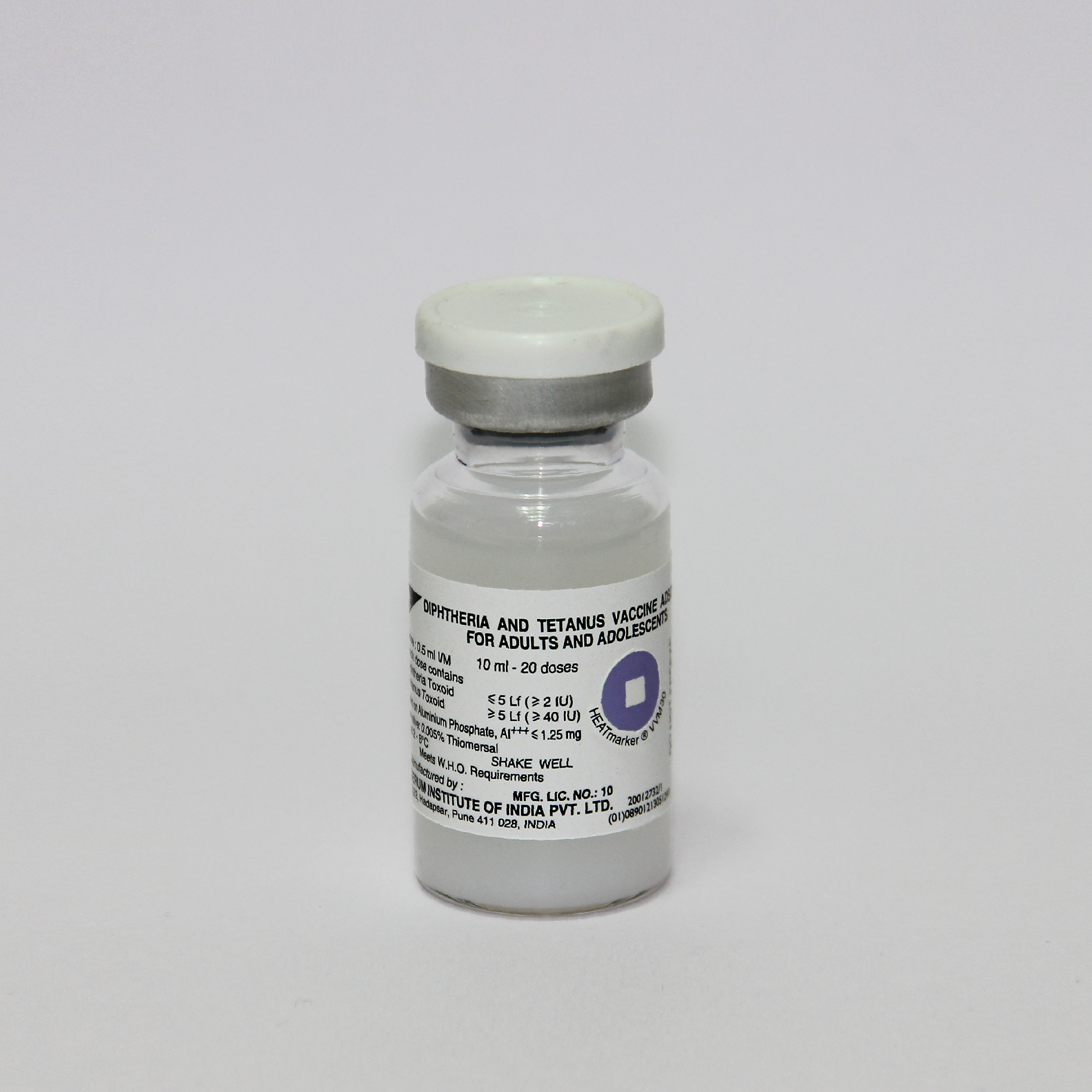
Diphtheria and Tetanus Vaccine Adsorbed for Adults and Adolescents
Diphtheria and tetanus vaccine (adsorbed, reduced diphtheria antigen content)
Product Details
WHO Product ID
FVP-P-123
Status
Prequalified
Date of prequalification
Vaccine type
Diphtheria and tetanus vaccine (adsorbed, reduced diphtheria antigen content)
Abbreviated Name
dT
Commercial Name
Diphtheria and Tetanus Vaccine Adsorbed for Adults and Adolescents
PQ Holder
Serum Institute of India Pvt. Ltd.
212/2 Off Soli Poonawala Road, Hadaspar
Pune,
Maharashtra
411 028
India
Responsible NRA
Central Drugs Standard Control Organization (CDSCO)
Product description
Pharmaceutical Form
Liquid: Ready to use
Presentation
Vial
Number of doses
20
Route of administration
Intramuscular
Vaccine Vial Monitor
Type 30
Multidose Vial Policy
WHO recommends that opened vials of this vaccine may be used in subsequent immunization sessions (up to 28 days) if the conditions outlined in the WHO Policy Statement are met.
Shelf life (months)
36 months
Storage temperature
2 - 8°C
Diluent
N/A
Preservative
Thiomersal
Preservative concentration
0.01%
Product Packaging
Secondary Container
Tertiary Container
Component Packed
Active (Vaccine)
Total doses
25
Description
carton of 25 vials (500 doses) [Dimensions 13.3x13.3x6.0 cm]
Dimensions
13.3 x 13.3 x 6.0 cm
Cold Chain Volume (cm³/dose)
2.12