WHO Product ID
TB085
Status
Prequalified
INN, dosage form and strength
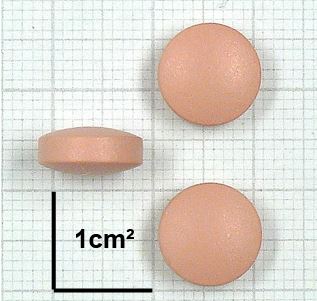
Isoniazid/Rifampicin Tablet, coated 75mg/150mg
Date of prequalification
Basis of listing
Prequalification - Abridged
Therapeutic area
Tuberculosis
Type
Finished Pharmaceutical Product
Dosage form
Tablet, coated
Applicant organization
Sandoz Pvt Ltd
K-3 MDT-TB, Plot No 8 A/2 & 8-B, TTC Industrial Area, MIDC, Kalwe Block, Dighe Village, Thane District
Navi Mumbai,
Maharashtra
400 708
India
Packaging details and storage conditions
Packaging Type
Blister, Alu/PVC/PE/PVdC
Configuration
28x4
Shelf life (months)
36
Storage conditions
Do not store above 25°C. Store in the original package to protect from moisture
API Manufacturing Site(s)
By Organization
By Active Ingredient
Chong Kun Dang (CKD) Bio Corporation
292, Sinwon-ro, Denwon-gu, Ansan-si, Gyeonggi-do
Seoul,
15604
Republic of Korea
Rifampicin
Yuki Gosei Kogyo Co Ltd
Joban Factory 788, Joban nishigo-machi, Iwaki-city
Fukushima,
972-8316
Japan
Isoniazid
FPP Manufacturing Site(s)
Panacea Biotech Pharma Limited
Malpur, Baddi, Solan District
Tehsil Nalagarh,
Himachal Pradesh
173 205
India
Sandoz Pvt Ltd
K-3 MDT-TB, Plot No 8 A/2 & 8-B, TTC Industrial Area, MIDC, Kalwe Block, Dighe Village, Thane District
Navi Mumbai,
Maharashtra
400 708
India