WHO Product ID
RH100
Status
Prequalified
INN, dosage form and strength
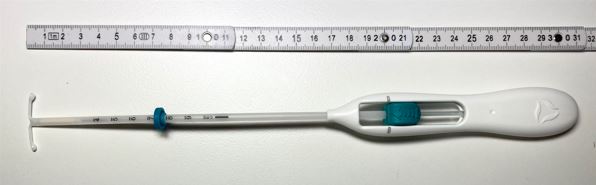
Levonorgestrel Intra-uterine system 52mg
Date of prequalification
Basis of listing
Prequalification - Abridged
Therapeutic area
Reproductive health
Type
Finished Pharmaceutical Product
Dosage form
Intra-uterine system
Administration Device
inserter (T-body, a plunger, flange, body and slider)
Applicant organization
Bayer AG
Müllerstraße 178
Berlin,
13353
Germany
Packaging details and storage conditions
Packaging Type
Blister, PETG/PE films 1x1 plus inserter (T-body, a plunger, flange, body and slider)
Configuration
1x1 plus inserter (T-body, a plunger, flange, body and slider)
Shelf life (months)
36
Storage conditions
Do not store above 30°C
API Manufacturing Site(s)
By Organization
By Active Ingredient
Bayer AG
Bergkamen Plant, Ernst Schering Strasse 14
Bergkamen,
59192
Germany
Levonorgestrel
FPP Manufacturing Site(s)
Bayer Oy
Pansiontie 47
Turku,
20210
Finland