WHO Product ID
RH087
Status
Prequalified
INN, dosage form and strength
Estradiol valerate/Norethisterone enantate Solution for injection 5mg/ml/50mg/ml
Date of prequalification
Basis of listing
Prequalification - Full
Therapeutic area
Reproductive health
Type
Finished Pharmaceutical Product
Dosage form
Solution for injection
Applicant organization
Bayer S.A.
Av. Nuestra Señora de Santa Ana E16-218 and N44C Leonardo Tejada Street. Ekopark
Quito,
170505
Ecuador
Packaging details and storage conditions
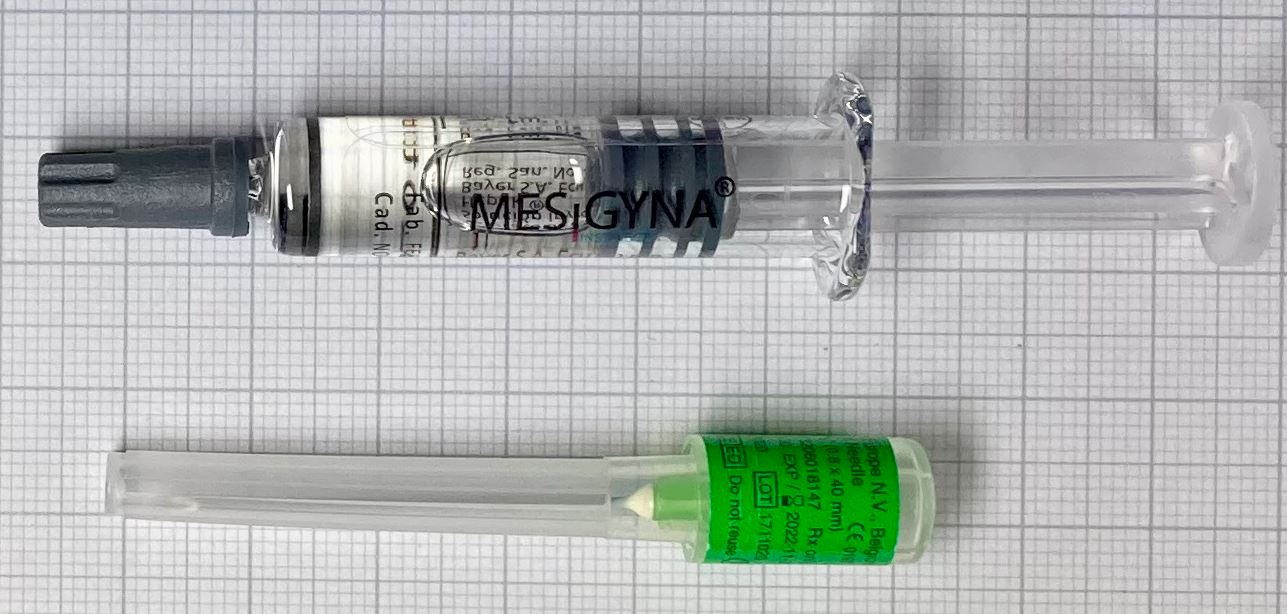
Packaging Type
Prefilled syringe, colorless glass type I, bromobutyl dark grey plunger
Configuration
1mlx1
Shelf life (months)
48
Storage conditions
Do not store above 30°C, protect from light, do not refrigerate or freeze
API Manufacturing Site(s)
By Organization
By Active Ingredient
Bayer AG
Bergkamen Plant, Ernst Schering Strasse 14
Bergkamen,
59192
Germany
Estradiol valerate, Norethisterone enantate
Bayer AG
Charlottenburg Plant, Max-Dohrn-Strasse 8
Berlin,
10589
Germany
Estradiol valerate
FPP Manufacturing Site(s)
Bayer de México S.A. de C.V.
Reforma Avenue No. 46 Col Potrerillo, 94450 Ixtaczoquitlán
Veracruz de Ignacio de la Llave,
Mexico