WHO Product ID
RH084
Status
Prequalified
INN, dosage form and strength
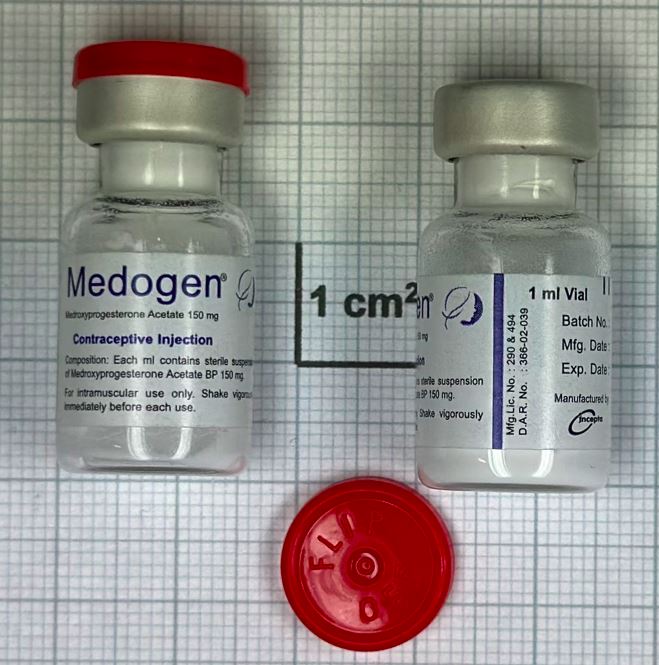
Medroxyprogesterone acetate Suspension for injection 150mg/ml
Date of prequalification
Basis of listing
Prequalification - Full
Therapeutic area
Reproductive health
Type
Finished Pharmaceutical Product
Dosage form
Suspension for injection
Applicant organization
Incepta Pharmaceuticals Ltd.
Unit – 1, Injectable Potent Drug (IPD), Krishnapura, Sahabelishor, Dhaka
Dhamrai,
Bangladesh
Packaging details and storage conditions
Packaging Type
Vial, Type I glass
Configuration
1x1; 1x20
Shelf life (months)
36
Storage conditions
Do not store above 30°C. Do not freeze. Store vials in the cartons to protect from light
API Manufacturing Site(s)
By Organization
By Active Ingredient
Farmabios S.p.A
Via Pavia 1, Gropello Cairoli
27027
Italy
Medroxyprogesterone acetate
FPP Manufacturing Site(s)
Incepta Pharmaceuticals Ltd.
Unit – 1, Injectable Potent Drug (IPD), Krishnapura, Sahabelishor, Dhaka
Dhamrai,
Bangladesh