WHO Product ID
RH079
Status
Prequalified
INN, dosage form and strength
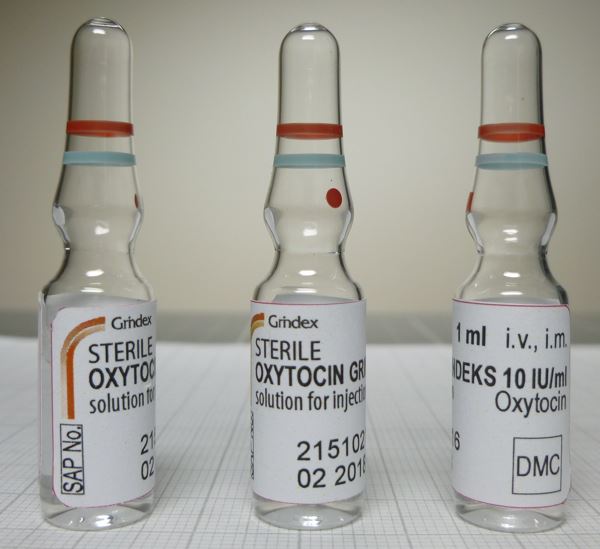
Oxytocin Solution for injection 10IU/ml
Date of prequalification
Basis of listing
Prequalification - Full
Therapeutic area
Reproductive health
Type
Finished Pharmaceutical Product
Dosage form
Solution for injection
Applicant organization
JSC Grindeks
53 Krustpils Street
Riga,
LV-1057
Latvia
Packaging details and storage conditions
Packaging Type
Ampoule, Type I glass
Configuration
1mlx5; 1mlx10; 1mlx100
Shelf life (months)
36
Storage conditions
Store in a refrigerator (2 °C to 8 °C), do not freeze
API Manufacturing Site(s)
By Organization
By Active Ingredient
JSC Grindeks
53 Krustpils Street
Riga,
LV-1057
Latvia
Oxytocin
FPP Manufacturing Site(s)
HBM Pharma s.r.o.
Sklabinska 30
Martin,
036 80
Slovakia
UAB Santonika
Veiveriu str. 134B
Kaunas,
LT- 46353
Lithuania