WHO Product ID
RH051
Status
Prequalified
INN, dosage form and strength
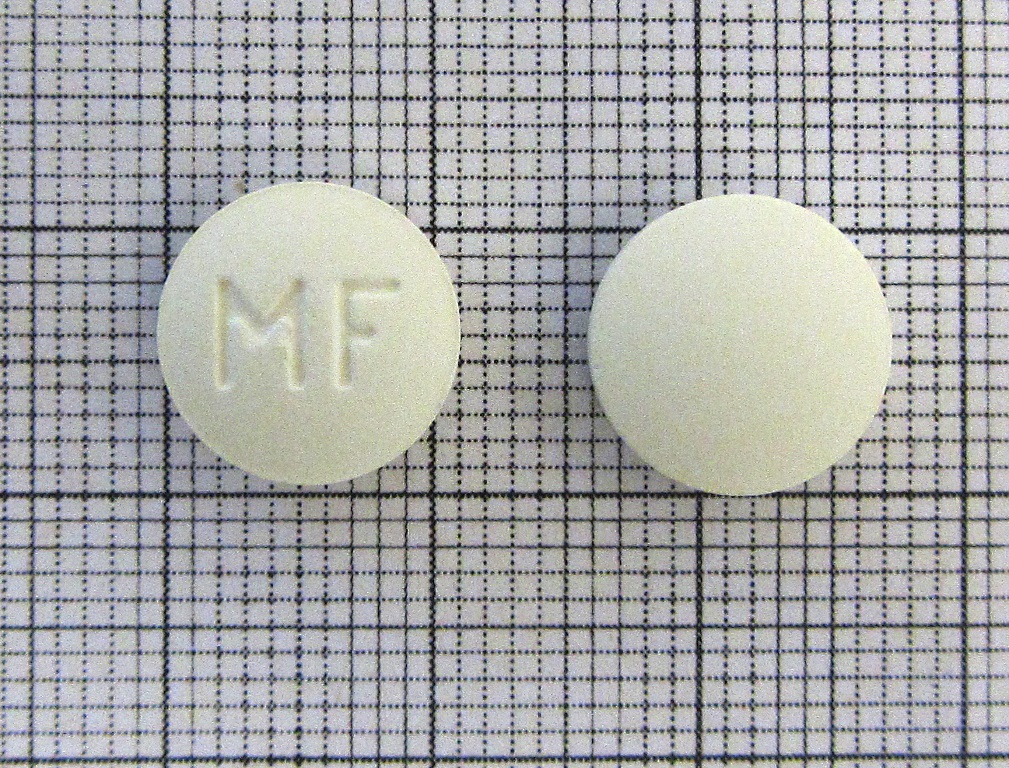
Mifepristone Tablet 200mg
Date of prequalification
Basis of listing
Prequalification - Abridged
Therapeutic area
Reproductive health
Type
Finished Pharmaceutical Product
Dosage form
Tablet
Applicant organization
Linepharma International Ltd
Regents Place, 338 Euston Road
London,
NW1 3BT
United Kingdom of Great Britain and Northern Ireland
Packaging details and storage conditions
Packaging Type
Blister, Alu/PVC/PVdC
Configuration
1x1, 1x30
Shelf life (months)
36
Storage conditions
Do not store above 25°C, protect from light
API Manufacturing Site(s)
By Organization
By Active Ingredient
Crystal Pharma SAU
Parque Tecnologico de Boecillo, Parcela 105
Boecillo,
47151
Spain
Mifepristone
FPP Manufacturing Site(s)
Centre Spécialités Pharmaceutiques (CSP)
76-78 avenue du Midi
Cournon d’Auvergne,
63800
France
Delpharma Lille SAS
Rue de Toufflers, ZI de Roubaix-Est
Lys-Lez-Lannoy,
59390
France
Laboratorios León Farma SA
C/La Vallina s/n, Polígono Industrial Navatejera, Villaquilambre
León,
24008
Spain