WHO Product ID
RH028
Status
Prequalified
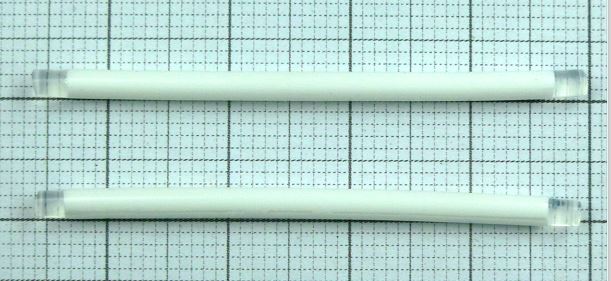
INN, dosage form and strength
Levonorgestrel Implant 75mg per rod (150mg in total)
Date of prequalification
Basis of listing
Prequalification - Full
Therapeutic area
Reproductive health
Type
Finished Pharmaceutical Product
Dosage form
Implant
Applicant organization
Shanghai Dahua Pharmaceutical Co Ltd
3503 Changzheng Road, Changzheng Farm, Chongming County
Shanghai,
Shanghai
China
Packaging details and storage conditions
Packaging Type
Pouch, PET/LDPE
Configuration
2 rods/pouchx10
Shelf life (months)
60
Storage conditions
Do not store above 30°C, protect from light
API Manufacturing Site(s)
By Organization
By Active Ingredient
Qinhuangdao Zizhu Pharmaceutical Co Ltd
No. 10 Longhai Road, Economic & Technological Development Zone
Qinhuangdao City,
Hebei
066 004
China
Levonorgestrel
FPP Manufacturing Site(s)
Shanghai Dahua Pharmaceutical Co Ltd
3503 Changzheng Road, Changzheng Farm, Chongming County
Shanghai,
Shanghai
China