WHO Product ID
RH022
Status
Prequalified
INN, dosage form and strength
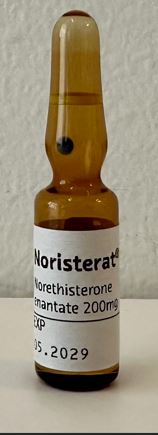
Norethisterone enantate Solution for injection 200mg/ml
Date of prequalification
Basis of listing
Prequalification - Abridged
Therapeutic area
Reproductive health
Type
Finished Pharmaceutical Product
Dosage form
Solution for injection
Applicant organization
Bayer plc
400 South Oak Way
Reading,
RG2 6AD
United Kingdom of Great Britain and Northern Ireland
Packaging details and storage conditions
Packaging Type
Ampoule, Glass
Configuration
1mlx100
Shelf life (months)
60
Storage conditions
Do not store above 30°C, protect from light
API Manufacturing Site(s)
By Organization
By Active Ingredient
Bayer AG
Bergkamen Plant, Ernst Schering Strasse 14
Bergkamen,
59192
Germany
Norethisterone enantate
FPP Manufacturing Site(s)
Bayer AG
Müllerstraße 178
Berlin,
13353
Germany