WHO Product ID
RH018
Status
Prequalified
INN, dosage form and strength
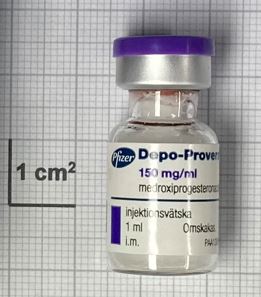
Medroxyprogesterone acetate Powder for suspension for injection 150mg/mL
Date of prequalification
Basis of listing
Prequalification - Abridged
Therapeutic area
Reproductive health
Type
Finished Pharmaceutical Product
Dosage form
Powder for suspension for injection
Applicant organization
Pfizer AB
113 63
Stockholm,
Sweden
Packaging details and storage conditions
Packaging Type
Vial, Glass
Configuration
1ml
Shelf life (months)
60
Storage conditions
Do not store above 25°C
API Manufacturing Site(s)
By Organization
By Active Ingredient
Pharmacia & Upjohn Co
7000 Portage Road
Kalamazoo,
MI 49001
United States of America
Medroxyprogesterone acetate
FPP Manufacturing Site(s)
Pfizer Manufacturing Belgium NV / SA
Rijksweg 12
Puurs Sint-Amands,
2870
Belgium