WHO Product ID
NT019
Status
Prequalified
INN, dosage form and strength
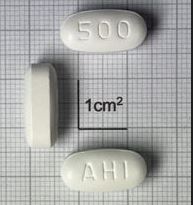
Azithromycin Tablet, Film-coated 500mg
Date of prequalification
Basis of listing
Prequalification - Abridged
Therapeutic area
Neglected tropical diseases
Type
Finished Pharmaceutical Product
Dosage form
Tablet, Film-coated
Applicant organization
ACI HealthCare Limited
Sonargaon Museum Gate-1 Road, Treepordi
Sonargaon,
Narayanganj
1440
Bangladesh
Packaging details and storage conditions
Packaging Type
Bottle, HDPE
Configuration
30x1
Shelf life (months)
36
Storage conditions
Do not store above 25°C
Packaging Type
Blister, • Alu-PVC/PE/PVDC
Configuration
3x1, 3x3
Shelf life (months)
24
Storage conditions
Do not store above 25°C
API Manufacturing Site(s)
By Organization
By Active Ingredient
Jiangsu Weiqida Pharmaceutical Co Ltd
No. 1 Linjiang Avenue, Linjiang Town, Haimen District
Nantong,
Jiangsu
226133
China
Azithromycin
FPP Manufacturing Site(s)
ACI HealthCare Limited
Sonargaon Museum Gate-1 Road, Treepordi
Sonargaon,
Narayanganj
1440
Bangladesh