WHO Product ID
NT017
Status
Prequalified
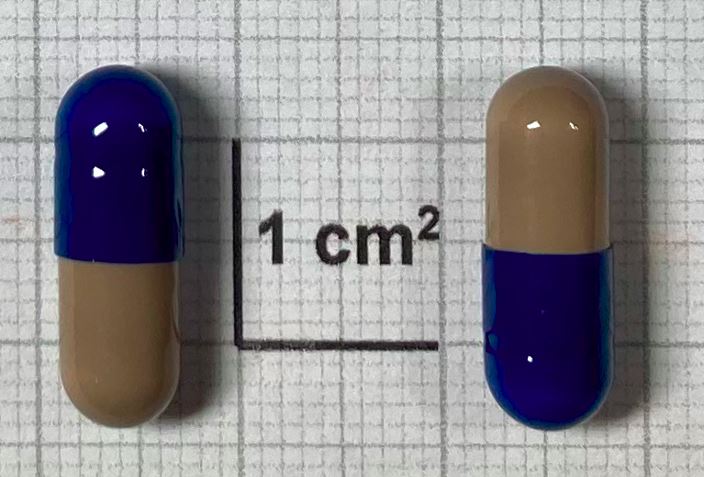
INN, dosage form and strength
Miltefosine Capsules, hard 50mg
Date of prequalification
Basis of listing
Prequalification - Full
Therapeutic area
Neglected tropical diseases
Type
Finished Pharmaceutical Product
Dosage form
Capsules, hard
Applicant organization
Zydus Lifesciences Limited
“Zydus Corporate Park”, Scheme no. 63, survey no. 536, Khoraj (Gandhinagar), Near. Vaishnodevi circle,
Ahmedabad,
Gujarat
382441
India
Packaging details and storage conditions
Packaging Type
Blister, Alu/Alu
Configuration
10x10
Shelf life (months)
36
Storage conditions
Do not store above 30°C, protect from moisture
API Manufacturing Site(s)
By Organization
By Active Ingredient
Zydus Lifesciences Limited
5/1-B, G.I.D.C., Industrial Estate, Bharuch
Ankleshwar,
Gujarat
393002
India
Miltefosine
FPP Manufacturing Site(s)
Zydus Lifesciences Limited
Kundaim Industrial Estate, Plot No.203-213, Kundaim
Goa
403115
India