WHO Product ID
MA200
Status
Prequalified
INN, dosage form and strength
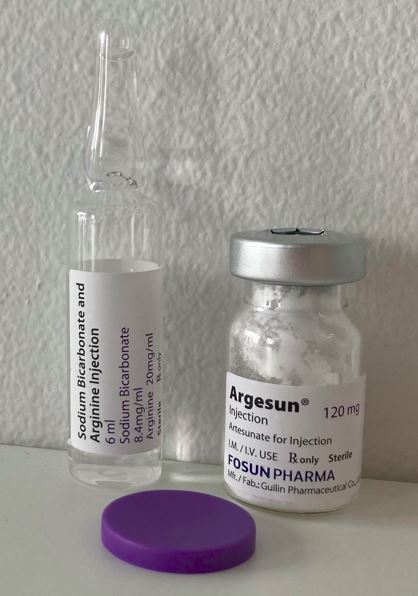
Artesunate Powder for solution for injection 120mg
Date of prequalification
Basis of listing
Prequalification - Full
Therapeutic area
Malaria
Type
Finished Pharmaceutical Product
Dosage form
Powder for solution for injection
Applicant organization
Guilin Pharmaceutical Co., Ltd.
INJ-I & INJ-II, No 43 Qilidian Road
Guilin,
Guangxi
541 004
China
Packaging details and storage conditions
Packaging Type
Vial, Type I glass + Ampoule, Type I glass
Configuration
120mg powder (Artesunate) in 7ml vial + 6ml solution (Arginine 20mg/ml+ Sodium bicarbonate 8.4mg/ml) in 6ml ampoule
Shelf life (months)
30
Storage conditions
Do not store above 30°C, protect from light. Avoid excursions above 30℃. Do not refrigerate or freeze
API Manufacturing Site(s)
By Organization
By Active Ingredient
Guilin Pharmaceutical Co., Ltd.
API-I No 43 Qilidian Road
Guilin,
Guangxi
541 004
China
Artesunate
FPP Manufacturing Site(s)
Guilin Pharmaceutical Co., Ltd.
INJ-I & INJ-II, No 43 Qilidian Road
Guilin,
Guangxi
541 004
China