WHO Product ID
MA177
Status
Prequalified
INN, dosage form and strength
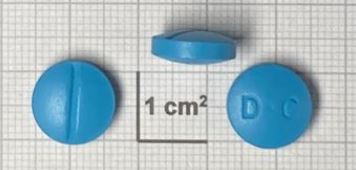
Dihydroartemisinin/Piperaquine phosphate Tablet, Film-coated 40mg/320mg
Date of prequalification
Basis of listing
Prequalification - Full
Therapeutic area
Malaria
Type
Finished Pharmaceutical Product
Dosage form
Tablet, Film-coated
Applicant organization
Beijing Holley-Cotec Pharmaceuticals Co Ltd
8th floor, Building 8, (3-3) 6 Langjiayuan, Chaoyang District
Beijing,
100022
China
Packaging details and storage conditions
Packaging Type
Blister, Alu/PVC
Configuration
6x1, 9x1, 6x2, 3x25, 6x25, 9x25 Blister(s), Strip(s), sealed in a PET/Al/PE pouch
Shelf life (months)
24
Storage conditions
Do not store above 25°C, protect from moisture, protect from light
API Manufacturing Site(s)
By Organization
By Active Ingredient
CR San Jiu (You Yang) Pharmaceutical Co., Ltd
108 Southern Jinyuan Road, Banxi Light Industry Area, Youyang
Youyang City,
Chongqing
China
Dihydroartemisinin
Mangalam Drugs and Organics Limited
Unit 1, Plot No 187, GIDC
Vapi,
Gujarat
396 195
India
Dihydroartemisinin
Zhuhai Rundu Pharmaceutical Co Ltd
No 6 North Airport Road, Sanzao Town, Zhuhai, Jinwan District
Guangdong
519 041
China
Piperaquine phosphate
FPP Manufacturing Site(s)
KBN-Zhejiang Pharmaceutical Co., Ltd.
340 Yunhai Road, Economy Development Zone
Jiaxing City,
Zhejiang
China