WHO Product ID
MA135
Status
Prequalified
INN, dosage form and strength
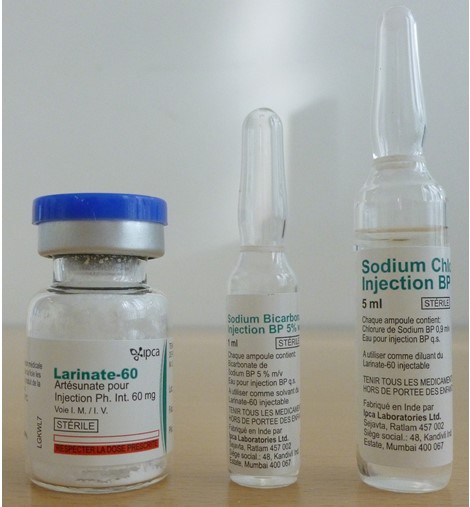
Artesunate Powder for solution for injection 60mg
Date of prequalification
Basis of listing
Prequalification - Full
Therapeutic area
Malaria
Type
Finished Pharmaceutical Product
Dosage form
Powder for solution for injection
Applicant organization
Ipca Laboratories Ltd
48 Kandivli Industrial Estate, Kandivli (West)
Mumbai,
Maharashtra
400 067
India
Packaging details and storage conditions
Packaging Type
Vial, type I glass + Ampoule, type I glass + Ampoule, type I glass
Configuration
60mg powder (Artesunate) in 5ml vial + 1ml solution (Sodium bicarbonate) in 2ml ampoule + 5ml solution (sodium chloride) in 5ml ampoule
Shelf life (months)
36
Storage conditions
Store below 30°C. Keep the vial and ampoules in the provided carton to protect the product from light. Do not refrigerate or freeze.
API Manufacturing Site(s)
By Organization
By Active Ingredient
Ipca Laboratories Ltd
Sejavta, Ratlam
Madhya Pradesh
457 001
India
Artesunate (sterile)
FPP Manufacturing Site(s)
Ipca Laboratories Ltd
P.O. Sejavta
Ratlam,
Madhya Pradesh
457 002
India