WHO Product ID
HA788
Status
Prequalified
INN, dosage form and strength
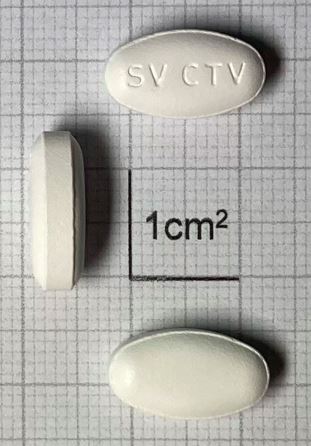
Cabotegravir (sodium) Tablet, Film-coated 30mg
Date of prequalification
Basis of listing
Prequalification - Abridged
Therapeutic area
HIV/AIDS
Type
Finished Pharmaceutical Product
Dosage form
Tablet, Film-coated
Applicant organization
ViiV Healthcare Pty Ltd
Packaging details and storage conditions
Packaging Type
Bottle, HDPE
Configuration
30x1
Shelf life (months)
48
Storage conditions
Do not store above 30°C
API Manufacturing Site(s)
By Organization
By Active Ingredient
Catalent Micron Technologies Inc.
Crossways Boulevard, Crossways, Dartford
Kent
DA26QY
United Kingdom of Great Britain and Northern Ireland
Cabotegravir (sodium)
Glaxo Operations UK Limited
Priory Street, Hertfordshire
Ware,
SG12 0DJ
United Kingdom of Great Britain and Northern Ireland
Cabotegravir (sodium)
Glaxo Wellcome Manufacturing Pte Ltd
1 Pioneer Sector 1, Jurong
628 413
Singapore
Cabotegravir (sodium)
FPP Manufacturing Site(s)
Glaxo Operations UK Limited
Priory Street, Hertfordshire
Ware,
SG12 0DJ
United Kingdom of Great Britain and Northern Ireland
FPP Packaging Only
Glaxo Wellcome SA
Avenue de Extremadura 3, Aranda de Duero
Burgos,
Spain