WHO Product ID
HA597
Status
Prequalified
INN, dosage form and strength
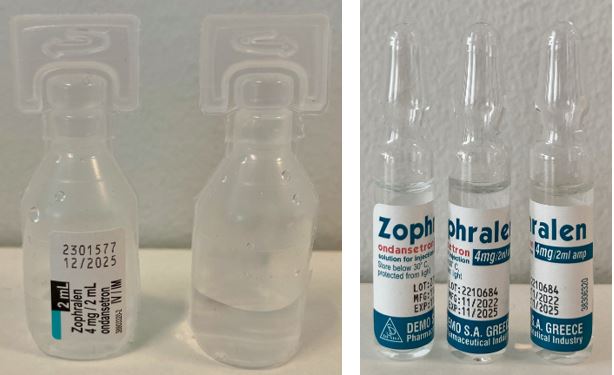
Ondansetron (hydrochloride) Solution for injection 4mg/2mL
Date of prequalification
Basis of listing
Prequalification - Abridged
Therapeutic area
HIV/AIDS
Type
Finished Pharmaceutical Product
Dosage form
Solution for injection
Applicant organization
Demo SA
21st Km National Road Athens-Lamia
Krioneri,
14568
Greece
Packaging details and storage conditions
Packaging Type
Ampoule, Glass
Configuration
2mlx5, 2mlx1
Shelf life (months)
36
Storage conditions
Do not store above 25°C
Packaging Type
Ampoule, PE
Configuration
2mlx5, 2mlx1
Shelf life (months)
36
Storage conditions
Do not store above 25°C
API Manufacturing Site(s)
By Organization
By Active Ingredient
Dr Reddy's Laboratories Ltd
Unit IV, Plot No 9A, I.D.A, Jeedimetla
Hyderabad,
Andhra Pradesh
500 055
India
Ondansetron (hydrochloride)
FPP Manufacturing Site(s)
Demo SA
21st Km National Road Athens-Lamia
Krioneri,
14568
Greece