WHO Product ID
HA530
Status
Prequalified
INN, dosage form and strength
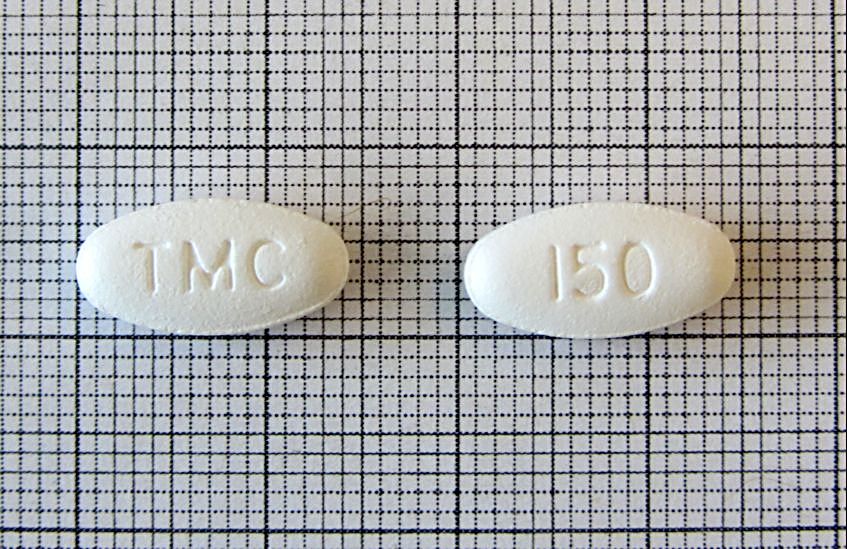
Darunavir (ethanolate) Tablet, Film-coated 150mg
Date of prequalification
Basis of listing
Prequalification - Abridged
Therapeutic area
HIV/AIDS
Type
Finished Pharmaceutical Product
Dosage form
Tablet, Film-coated
Applicant organization
Janssen Research & Development LLC
Turnhoutseweg 30
Beerse,
2340
Belgium
Packaging details and storage conditions
Packaging Type
Bottle, HDPE
Configuration
240x1
Shelf life (months)
36
Storage conditions
Do not store above 30°C
API Manufacturing Site(s)
By Organization
By Active Ingredient
Cilag AG
Hochstrasse 201
Schaffhausen,
8205
Switzerland
Darunavir (ethanolate)
Janssen Pharmaceutical Sciences Unlimited Company (JPSUC)
Little Island Industrial Est.
Little Island,
Ireland
Darunavir (ethanolate)
Porton Pharma Solutions Ltd
1 Fine Chemical Zone, Chongqing, Chemical Industry Park
Changshou,
Chongqing
401221
China
Darunavir (ethanolate)
FPP Manufacturing Site(s)
Janssen Ortho LLC
State Road 933 KM 0.1, Mamey Ward
Gurabo,
Puerto Rico
00778
United States of America
Janssen-Cilag SpA
Via C Janssen, Borgo San Michele
Latina,
4100
Italy