WHO Product ID
HA479
Status
Prequalified
INN, dosage form and strength
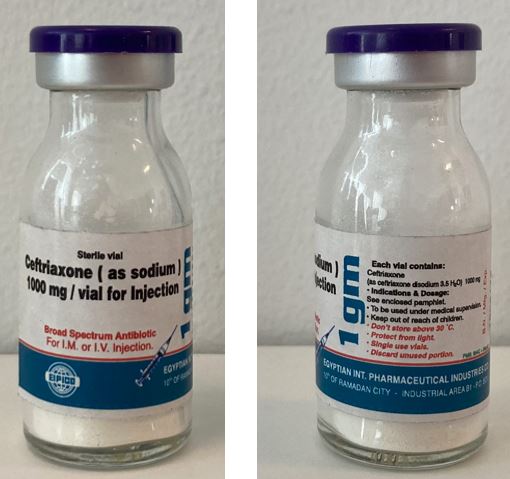
Ceftriaxone (sodium) Powder for solution for injection 1000mg
Date of prequalification
Basis of listing
Prequalification - Full
Therapeutic area
HIV/AIDS
Type
Finished Pharmaceutical Product
Dosage form
Powder for solution for injection
Applicant organization
Egyptian International Pharmaceutical Industries
PO Box 149
10th of Ramadan City,
Egypt
Packaging details and storage conditions
Packaging Type
Vial, Glass Type II
Configuration
1x1
Shelf life (months)
36
Storage conditions
Do not store above 30°C, protect from light
API Manufacturing Site(s)
By Organization
By Active Ingredient
Fresenius Kabi IPSUM S.R.L.
Via San Leonardo 23
Villadose,
Rovigo
45010
Italy
Ceftriaxone
FPP Manufacturing Site(s)
Egyptian International Pharmaceutical Industries
Cephalosporin Block, Industrial Area B1
10th of Ramadan City,
Egypt