Product Details
WHO Product ID
HA096
Status
Prequalified
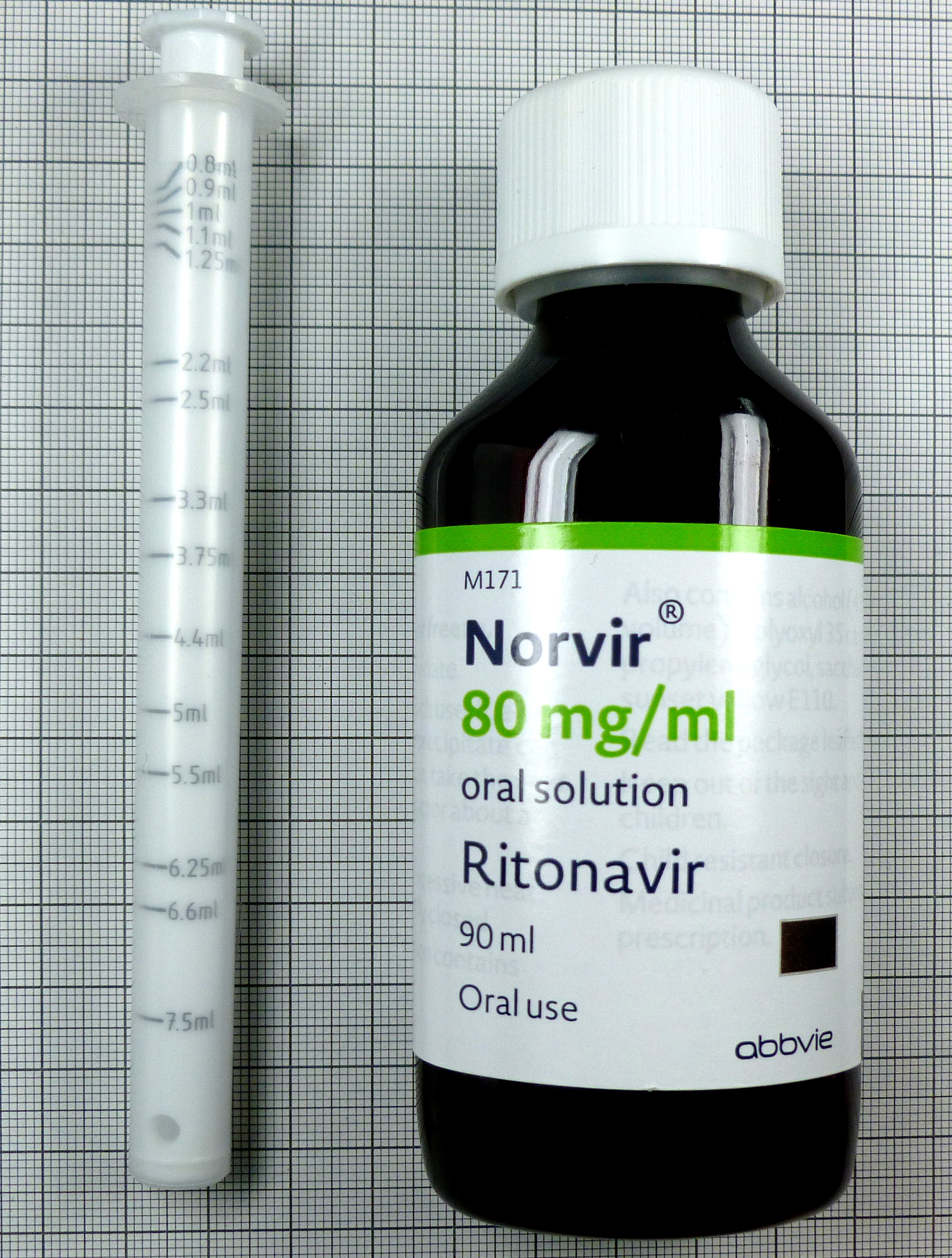
INN, dosage form and strength
Ritonavir Solution, Oral 80mg/mL
Date of prequalification
Basis of listing
Prequalification - Abridged
Therapeutic area
HIV/AIDS
Type
Finished Pharmaceutical Product
Dosage form
Solution, Oral
Applicant organization
AbbVie Ltd
AbbVie House, Vanwall Road
Maidenhead,
SL6 4UB
United Kingdom of Great Britain and Northern Ireland
Packaging details and storage conditions
Packaging Type
Bottle, PET
Configuration
90mlx1
Shelf life (months)
6
Storage conditions
Do not store above 25°C
API Manufacturing Site(s)
AbbVie S.r.L
S.R. 148 Pontina, Km 52 snc
Campoverde di Aprilia (LT),
4011
Italy
FPP Manufacturing Site(s)
Aesica Queenborough Limited
North Road
Queenborough,
ME11 5EL
United Kingdom