WHO Product ID
CV020
Status
Prequalified
INN, dosage form and strength
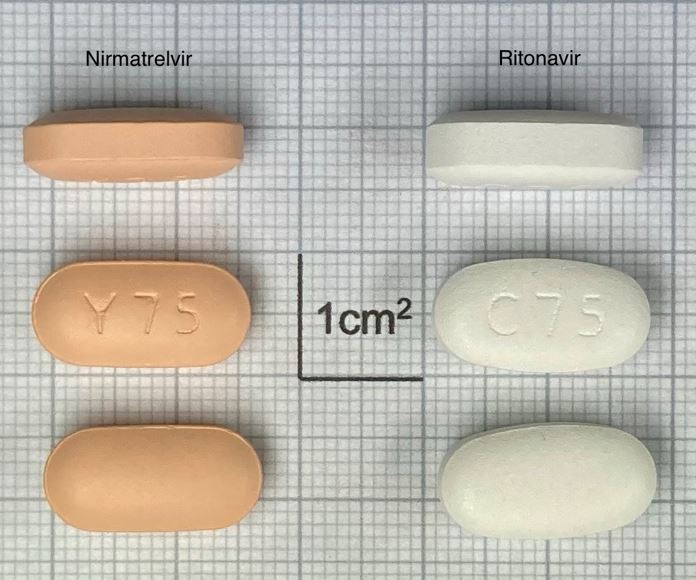
Nirmatrelvir Tablet, Film-coated + Ritonavir Tablet, Film-coated 150mg + 100mg
Date of prequalification
Basis of listing
Prequalification - Full
Therapeutic area
Covid-19
Type
Finished Pharmaceutical Product
Dosage form
Tablet, Film-coated + Tablet, Film-coated
Applicant organization
Yaopharma Co., Ltd.
100 Xingguang Avenue, Renhe Town, Yubei District, Factory Bldg. No. 2, Oral solid line I
Chongqing
401121
China
Packaging details and storage conditions
Packaging Type
Blister: Alu/PA/Alu/PVC
Configuration
(4 Nirmatrelvir + 2 Ritonavir) x 5
Shelf life (months)
24
Storage conditions
Do not store above 30°C
API Manufacturing Site(s)
By Organization
By Active Ingredient
Chongqing Carelife Pharmaceutical Co., Ltd.
No. 2, The Third Branch Road, Huanan Road
Yan Jia, Changshou District,
Chongqing
401221
China
Nirmatrelvir
Shanghai Desano Chemical Pharmaceutical Co., Ltd.
417 Binhai Road, Laogang Town, Pudong New Area
Shanghai
201302
China
Ritonavir
FPP Manufacturing Site(s)
Yaopharma Co., Ltd.
100 Xingguang Avenue, Renhe Town, Yubei District, Factory Bldg. No. 2, Oral solid line I
Chongqing
401121
China