WHO Product ID
BT-ON002
Status
Prequalified
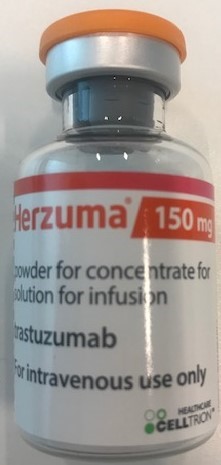
INN, dosage form and strength
Trastuzumab Powder for concentrate for solution for infusion 150mg
Date of prequalification
Basis of listing
Prequalification - Abridged
Therapeutic area
Oncology
Type
Biotherapeutic Product
Dosage form
Powder for concentrate for solution for infusion
Applicant organization
Celltrion Inc.
20, Academy-ro 51 beon-gil
Yeonsu-gu,
Incheon-gwangyeoksi
22014
Republic of Korea
Packaging details and storage conditions
Packaging Type
Vial, 20 mL clear glass type I
Configuration
1x1
Shelf life (months)
60
Storage conditions
Store in a refrigerator (2 °C to 8 °C)
FPP Manufacturing Site(s)
Celltrion Inc.
20, Academy-ro 51 beon-gil
Yeonsu-gu,
22014
Republic of Korea