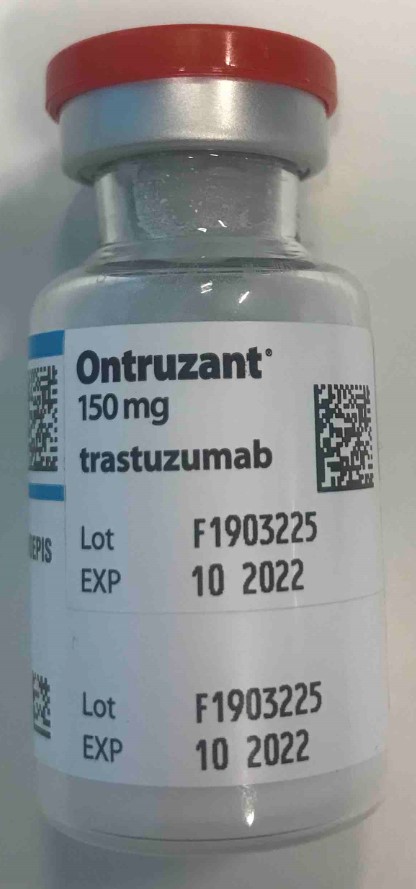
WHO Product ID
BT-ON001
Status
Prequalified
INN, dosage form and strength
Trastuzumab Powder for concentrate for solution for infusion 150 mg
Date of prequalification
Basis of listing
Prequalification - Abridged
Therapeutic area
Oncology
Type
Biotherapeutic Product
Dosage form
Powder for concentrate for solution for infusion
Applicant organization
Samsung Bioepis NL B.V.
Olof Palmestraat 10
Delft,
2616 LR
Netherlands
Packaging details and storage conditions
Packaging Type
Vial, 15ml type I borosilicate glass
Configuration
1X1
Shelf life (months)
48
Storage conditions
Store in a refrigerator (2 °C to 8 °C)
FPP Manufacturing Site(s)
Biogen Manufacturing ApS
Biogen Allé, 1
Hillerød,
3400
Denmark