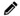
Title
Why
The first step in standardizing the primary process is the documentation of the procedures of all the tests currently performed in the laboratory in Standard Operating Procedures (SOPs).
What
Develop SOPs for all the tests identified in the list made earlier in phase 1. Do this according to the protocol for writing Analysis SOPs in the Master SOP developed in the previous activity and use the template for an Analysis SOP attached to the Master SOP.
One important remark is that the SOPs for the different tests can best be written by the staff members who most often perform the test. These staff members know best all the small details that make the tests perform optimally and can describe these in the SOPs. Spreading the tasks of writing SOPs over different staff members moreover prevents the overloading of one staff member with a huge amount of work and increases the involvement and commitment of all staff members.
In the right-hand column an example of an Analysis SOP is attached. This can be used to get an impression of the writing style and the level of detail of the text.
For your information:
Several portals exist that provide generic SOP templates for many tests often performed in public health laboratories. Below several links to these portals are provided. It is however extremely important to keep in mind that these generic SOPs always need to be checked and adapted to the situation in your laboratory. If this is not done, the procedure described in the generic SOP may be different from the procedure as performed in your laboratory. To achieve standardization and assure quality you have to write what you do and do what you write. If the generic SOPs are not checked and adapted to the situation in your laboratory, the procedures performed in your laboratory are still not standardized and quality is not assured.
- The Patient Safety Monitoring & International Evaluation Project (pSMILE) of the John Hopkins University provides a portal with resources for laboratories. This portal contains many different types of documents that can be used in laboratory practice and for auditing laboratories. Among these documents are SOPs for laboratory tests. Click here to go to this portal.
- The Caribbean Med Labs Fountation has published many microbiology SOPs on its website (in PDF). Click here.
- Tuberculosis laboratories can use the TB CAP Laboratory Toolbox which contains many SOP templates specific for tuberculosis tests. Download this toolbox here. [TB] Find the SOPs for TB tests on the right of this page.
A lot of organizations have published their SOPs on the internet; you can use these as “inspiration” for writing your own SOPs.
How & who
Quality Project Team in cooperation with the Laboratory Manager:
- Determine which staff members are most suitable to write each of the SOPs.
- Laboratory Manager:
- Assign the selected staff members to write the SOPs. Explain the procedure of writing SOPs and tell them to familiarize themselves with the Master SOP and use the protocol for writing Analysis SOPs in the Master SOP and using the template for an Analysis SOP attached to the Master SOP. Explain that if they have questions they can go to a member of the Quality Project Team.
- Clearly define the tasks of the staff member (creating the SOP content: writing the procedure and other paragraphs of the SOPs) and of the Quality Project Team (optimizing the SOP structure and lay-out to make it complying with the Master SOP guidelines. This way everybody knows what to do and double efforts are prevented.
- Make SMART appointments with the different staff members when to finish their tasks to keep this process going.
- When SOPs are ready (i.e. written, reviewed, and authorized) determine at which location copies of the SOPs must be available and indicate these locations on the SOP to keep track of this and to know where to replace old copies when the SOPs are revised. Make folders entitled "Analysis SOPs" for storing the SOPs at the various locations identified.
- Include the SOPs in a Read and Understand List and indicate which staff members must read and understand the SOPs (those for whom the SOPs are relevant). A template for a Read and Understand List is provided in the right-hand column.
- Present the SOPs and where to find them in a weekly staff meeting to all the staff members. Explain which staff members have to read the SOPs and explain about the Read and Understand List and that they must sign the list when they have read an SOP.
- Monitor that all the staff members have read the SOPs assigned to them. If not, remind them that they still have to do this.