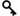
Title
Why
Achievement of goals set at the beginning of the quality year must be monitored and reviewed by the Laboratory Manager. This ensures continuity in the implementation, maintenance and improvement of the quality management system.
What
The management review is done by the Laboratory Manager. During the management review the Laboratory Manager focuses on/reviews:
- Performance data and/or information arising from internal audits and nonconformity management
- Review of major changes in operation of the laboratory
- Verification that the quality management system is implemented correctly and according to schedule
- Feedback (complaints, satisfaction surveys, etc.) from staff and clients
- Status of current continual improvement activities
- Identification of new opportunities for improvement, including selection and prioritization of these opportunities considering availability of resources
- Allocation of resources needed for follow-up actions from the management review
The following elements can serve as input for the management review:
- Follow-up of actions originating from the last management review (one year ago)
- Achievement of goals set in the Quality Year Plan
- Result of the selection and assessment of suppliers to the laboratory
- Assessment of adherence to turnaround times
- The competence of personnel (competency assessments) and their training needs
- Reports of external quality assessments
- Reports of internal and external quality audits and the follow-up
- Recommendations for improvement
- Quarterly quality reports
- Complaint forms
- ...
The findings of the management review are recorded in the Management Review Report. This report describes per reviewed topic:
- Desicions made
- A description of discussion/argumentation behind the decisions made
- Follow-up actions
- Conclusion
An Example Management Review is provided in the right-hand column. The example is a Management Review report of a National Tuberculosis Reference Laboratory, however, the principle of performing a management review is the same for any other type of laboratory. The management review report serves as input for the new Quality Year Plan of the following year.
NOTE: the management review is traditionally done once per year. However, during the introduction of the quality management system it may be more convenient to do a management review twice per year.
How & who
Laboratory manager:
- Organize the management review, make sure that all key personnel and relevant documents are present.
- Record the findings of this review in a Management Review Report. Include the evidence for completion of action plans. Create a logical outline (an option could be to follow the Quality System Essentials framework). If the review has led to suggestions for improvement, write these down in the form of SMART action points.
- Sign and date the report after finalization. Submit the report to the Quality Officer for follow-up and archiving.
- Present the findings of the management review to all laboratory staff in a weekly staff meeting and discuss action points yielding from the Management Review.
- Document the procedure of performing a management review in an SOP.
Quality Officer:
- Store the Management Review Report in the Management Reviews and Quality Year Plans folder behind the appropriate tab after discussion in the weekly staff meeting.
- Monitor the implementation of the action plan through Quarterly Reports and by adding the action points to the minutes of the weekly staff meeting. This way you can go through the action points every staff meeting and ask for their status of implementation. As the laboratory manager also attends these meetings the staff members that have not yet implemented action points after the deadlines can be forced to implement the action points as soon as possible by the laboratory manager.