Title
Why
The environment and equipment can have a considerable effect on many examinations and reagents. Many reagents have a minimum and maximum storage temperature, bacteria have an optimum growth temperature, negative pressure in a laboratory should be constant and within a specified interval, etc. When too much fluctuations in these parameters occur, examinations are negatively influenced, reagents may be damaged/expire earlier, bacterial growth may be slower. Parameters of equipment and the environment that could have an influence on the examination should thus be identified, monitored and controlled.
What
In this activity the first two steps are taken in controlling environmental parameters:
- Identification of critical parameters
- Setting up monitoring systems.
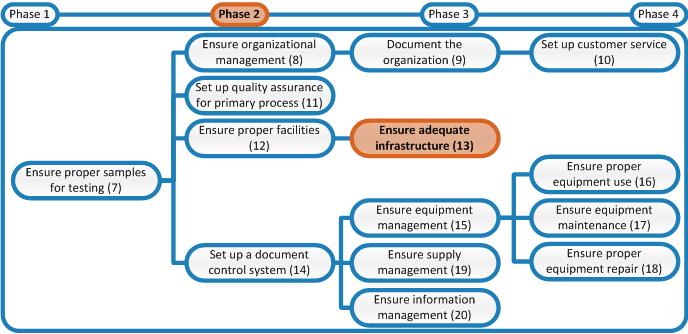
In phase 4 a standardized procedure for nonconformity handling will be established that will lead to standardized preventive- and corrective actions. At that moment the third step, controlling of these parameters, will be implemented.
How & who
Biosafety Officer:
- Make a list of parameters that could have an influence on reagents, equipment or the examination. Examples of critical parameters:
- Temperature of incubators
- Temperature of refrigerators and freezers
- Environmental temperature
- Environmental pressure differential between the laboratory and the external environment (in case a negative pressure system is in place)
- Calibration of equipment
- Other parameters if applicable and necessary (i.e. when deviation from standard norms negatively influences test results)
- Define for each parameter the critical interval. This means that when the parameter has a value above or below this interval, a corrective action is necessary to correct the parameter and bring it back to normal values.
- Define for each parameter the frequency with which it should be monitored (e.g. daily/weekly/monthly).
- Discuss the parameters with the Laboratory Manager. The Laboratory Manager must decide which person(s) will be assigned with the task of monitoring the various parameters.
- Develop for each parameter a monitoring log sheet that will be put on the wall near the indicator of each parameter. Include the critical interval and the frequency of measurement on this log sheet! NOTE that these log sheets must be included in the relevant SOPs. Most SOPs will be written in this phase so there are two possibilities:
- The SOP has already been written: add the monitoring log sheet as appendix to this SOP and adapt the SOP to include the procedure for regularly monitoring the respective parameter.
- The SOP still needs to be written: Already make the log sheet and start using it. Wait for the moment of writing the SOP to describe the procedure for regularly monitoring this parameter.
- Explain to all staff members in a Weekly Staff Meeting the procedure and reason for monitoring critical parameters.
- Start monitoring critical parameters.
- Regularly check if log sheets need to be replaced. Store the completed log sheets in a new folder entitled: "Critical Parameter Monitoring".
- After some time, check if the monitoring of the parameters is done consequently and correctly. If not, discuss this with the Laboratory Manager and take appropriate actions.
Laboratory manager:
- Assist the Biosafety Officer where necessary.
- Assign the tasks of monitoring parameters to several staff members. Choose the most relevant staff member, i.e. those most closely involved. For example: personnel working in the bacteriology laboratory will be the most suitable persons to monitor the temperature of incubators and fridges used for bacteriology.
- Discuss with these staff members their new task.
In the right-hand column there are several templates of log sheets that could be adapted for use in your laboratory. These log sheets must included as part of the equipment SOP for the pieces of equipment for which they were developed, for example, as an annex to the relevant SOP. These templates were adapted with permission from resources within the TB CAP Laboratory Toolkit, the development of which was funded by USAID. The originals and other useful documents may be found here in the TB CAP Laboratory Toolbox