Title
Why
Implementing a quality management system is a long process with many activities in many different areas of laboratory practice. Coordinating this process is a big task that cannot be done by one person alone. More people -joined together in a team- are needed to coordinate, plan, manage and execute the implementation of quality management.
What
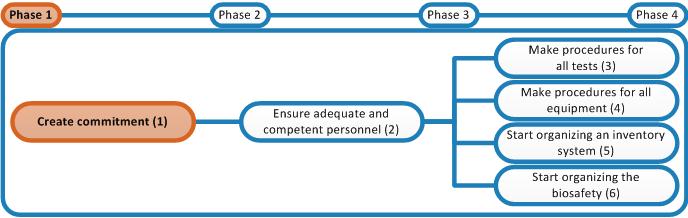
In this tool the appointment of a Quality Project Team is chosen as a strategy to tackle the amount of work that needs to be done. This team is the main coordinating body for implementing the quality management system. It will support the management in carrying out the activities described in this tool.
The chair of the Quality Project Team will be appointed as the Quality Focal Point of the laboratory. This can be considered as a Quality Officer only with less responsibilities and tasks (the "real" Quality Officer will be appointed in phase 2). The Quality Focal Point will closely follow correct implementation of this tool. His/her functions are:
- Coordination function: ensuring correct and timely execution of activities of the tool by team members.
- Advisory function: regularly report to the Laboratory Manager on the progress of implementing the quality management system, and cooperate with the Laboratory Manager where necessary in the execution of activities in which there are specific tasks for the laboratory manager.
- Information function: laboratory staff can turn to this person for more information on quality management aspects. The other way around is also possible: the Quality Focal Point can provide information to staff members regarding the quality management system.
The tasks and responsibilities of Quality Project Team members are:
- Execution of activities: Carry out activities/tasks assigned to them by the Quality Focal Person. Involve other staff members of the laboratory in carrying out these activities. For example: when a Standard Operating Procedure (SOP) on Recording and Reporting of Results needs to be written, the team member should closely cooperate with a data clerk to write an SOP.
- Advising/reporting: the team member must report to the Quality Project Team about the status of the activities assigned to him/her.
- Planning: The team member must actively participate in the coordination of the quality management system implementation and should assist in the planning of activities during the team meetings.
This team will have meetings at least twice per month to discuss progress and find solutions to problems encountered. The Laboratory Manager must participate in these meetings but the meetings are chaired by the Quality Focal Point. Minutes of these meetings are made by one member of the Quality Project Team (not the Quality Focal Person since he/she has to chair the meetings). These minutes must be distributed to all members of the project team and the Laboratory Manager and they must be archived (i.e. they serve as proof that the meetings were held and they serve as tool to monitor progress).
How & who
Laboratory Manager:
- Decide how many persons the Quality Project Team must consist of, compared to the amount of work that needs to be done (this depends on the size of the laboratory) and the laboratory's deadline for receiving accreditation. However, be mindful that the group should be small with a maximum of 6 persons to keep the situation manageable and workable. Experience has shown that too many people in this team can lead to complications and delays.
- Appoint staff members as members of the Quality Project Team. Include the Biosafety Officer who will also be appointed in this phase. Appoint a chair of this team; this will be the Quality Focal Person of the laboratory.
- Take into account that being member of the Quality Project Team will take time that the staff members can't spend on doing their regular work. This is especially the case for the Quality Focal Person. If needed, hire new staff or reorganize the laboratory (if possible) to prevent overburdening of Quality Project Team members (or their colleagues).
- Explain to the Quality Project Team members and the Quality Focal Person what their tasks and responsibilities are.
- Schedule the first meetings of the team (at least twice per month but more often if convenient).
- Monitor the work of the team. Help to solve problems if these occur and take care that the team keeps functioning correctly.
Quality Focal Point:
- Lead the team meetings and appoint one team member who has to write minutes of these meetings.
- Monitor the progress of the activities (discuss action points in the minutes, see below).
- Inform the Laboratory Manager about the developments.
Quality Project Team member assigned with the task to write minutes:
- Make minutes of each meeting and distribute these to the rest of the team within one day after the meeting. They should at least consist of the following parts:
- Summary of what was discussed during the meeting.
- Overview of SMART action points (click here to go to the activity on making SMART action points).
- Agenda for the next meeting, including the planned date of the next meeting.
- Take a new folder and name this "Minutes".
- Insert a tab entitled "Quality Project Team Minutes" and archive the minutes in this folder behind this tab.
NOTE: a template of minutes is added in the right-hand column that can be used for writing minutes of the Quality Project Team meetings. Feel free to adapt this template to suit your demands and wishes.