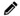
Title
Add a front page to each quality document used in the laboratory
Why
Each quality document in the laboratory will be managed in a document control system that will be established in the next activity. In order to manage the documents correctly they need to be supplied with metadata that can be used to identify the document, the version of the document, the author, reviewers, and authorizers, and the locations where copies of the document can be found.
What
Design a front page that can be added to each document and that contains all the metadata that are needed for the document control system. The front page must contain at least the following information:
- Document code
- The document title
- The name of the author
- The names of the reviewers
- The name of the authorizer
- The current version number
- The date on which it was released for use
- The date before which the document must be reviewed
- Distribution list (locations where authorized versions can be found)
- Description of changes compared to previous version
In the right-hand column a template of the front page is provided.
How & who
Quality Officer:
- Design the front page to be used in your laboratory. You can use the template provided in the right-hand column as the basis.
- Add a front page to each quality document in the laboratory. This means each SOP, biosafety chapter, and quality manual chapter (when these will be written in phase 3). You don't have to add a front page to each appendix or form that is part of a quality document because the same information that applies to the quality document also applies to the appendixes and forms that are part of that document. You can copy the information for each quality document from the document control log made in the previous activity.