Title
Why
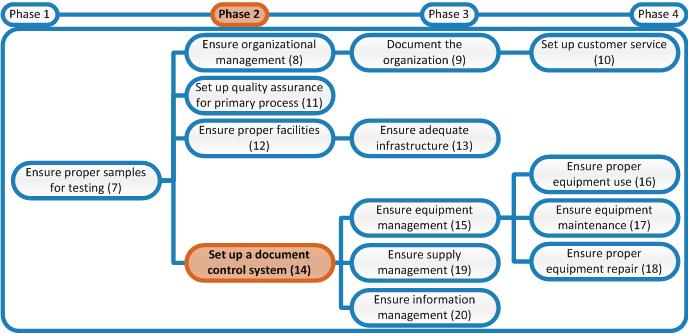
Later in phase 2 a document control system needs to be established. This system ensures that all documents are regularly reviewed and, if necessary, revised. To keep oversight a document control log is necessary.
In the right-hand column links to parts of the WHO Laboratory Quality Management System (LQMS) handbook are provided which provide general information on document control and an overview of the different types of records that are present in the laboratory.
What
Develop a document control log. This is an overview of all the documents maintained in the document control system, which are:
- SOPs, including their appendixes and forms
- Quality Manual chapters and appendixes (to be developed in phase 3)
- Biosafety Manual chapters and appendixes
Of each document the document control log provides the following metadata:
- The document code
- The document title
- The name of the author
- The names of the reviewers
- The name of the authorizer
- The current version number
- The date on which it was released for use
- The date before which the document must be reviewed
- Distribution list
- Remarks
- The title of the previous version
- The name of the author of the previous version
- The names of the reviewers of the previous version
- The name of the authorizer of the previous version
- The date on which the previous version was released for use
- The date on which the previous version was replaced
Regarding forms and appendixes of SOPs, quality manual, Laboratory Service Manual and Biosafety Manual, the laboratory has a choice:
- The forms and appendixes can be seen as an integral part of the main documents: the advantage is that the Document Control Log remains less extensive. The disadvantage is that when a form must be adapted (e.g. when an error in the form is discovered), the complete document with appendixes, including the form, needs to be reprinted and redistributed. This is a waste of paper, printer ink and time.
- The forms and appendixes of documents can be inserted separately from the main document in the Document Control Log. The disadvantage is that the document control log becomes larger this way. The advantage is that the form or the appendix of a document can be adapted if an error is discovered without having to reprint the complete document again. Note that when a main document needs to be reviewed, all the forms and appendixes attached to this document need to be reviewed at the same time, even though they have a different version number and are mentioned separately from the main document in the Document Control Log.
A template for a document control log is provided in the right-hand column.
How & who
Quality Officer:
- Create the document control log according to the description above and the template Document Control Log provided in the right-hand column.