Title
Why
To ensure that equipment is operated and maintained correctly at all times, you have to write Standard Operating Procedures (SOPs) on using and maintaining equipment.
What
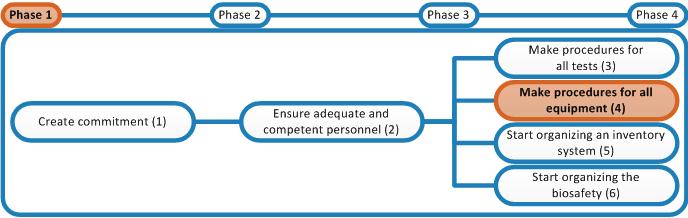
In the previous activity all the equipment of the laboratory was introduced in a register. Determine for which pieces of Equipment SOPs need to be written. Not all the equipment present in the laboratory needs to be supplied with an SOP. E.g. simple equipment such as pipettes and clocks don't need SOPs for correct operation. However, centrifuges are already more complicated machines that need correct programming and handling. For this SOPs definitely need to be written. You have to determine yourself which equipment needs to be supplied with an SOP. Write SOPs in accordance with the procedure of writing Equipment SOPs described in the Master SOP.
To give you a kick start an example Equipment SOP has been provided in the right-hand column to give you an idea of the general writing style and content of the SOPs.
Also several portals exist that provide generic SOP templates for equipment often used in public health laboratories. Below several links to these portals are provided. It is however extremely important to keep in mind that these generic SOPs always need to be checked and adapted to the situation in your laboratory. If this is not done, the procedure described in the generic SOP may be different from the procedure as performed in your laboratory. To achieve standardization and assure quality you have to write what you do and do what you write. If the generic SOPs are not checked and adapted to the situation in your laboratory, the procedures performed in your laboratory are still not standardized and quality is not assured.
- The Patient Safety Monitoring & International Evaluation Project (pSMILE) of the John Hopkins University provides a portal with resources for laboratories. This portal contains many different types of documents that can be used in laboratory practice and for auditing laboratories. Among these documents are SOPs for laboratory equipment. Click here to go to this portal.
- Tuberculosis laboratories can use the TB CAP Laboratory Toolbox which contains many SOP templates of Equipment SOPs specific for tuberculosis laboratories. Download this toolbox here. You will find on the right some examples of SOPs for the equipment commonly used in laboratories.
A lot of organizations have published their SOPs on the internet; you can use these as “inspiration” for writing your own SOPs.
How & who
Quality Project Team/Quality Focal Point in cooperation with the Equipment Officer and the Laboratory Manager:
- Make a list of SOPs needed.
- Determine who must write the SOPs. In most cases it can be the Equipment Officer. However, when a specific colleague is the one most often operating one specific piece of equipment, it may be better if this person writes the SOP for that piece of equipment in cooperation with the Equipment Officer.
Quality Project Team/Quality Focal Point:
- Make a planning for writing the SOPs.
- When colleagues have to write certain SOPs guide them in this process to make sure that the SOPs are written correctly and are complete (in concordance with the Master SOP also developed in phase 1). Note that when equipment needs regular calibration, the SOP must also describe this procedure.
- When drafts of SOPs have been written, forward a copy to one or two colleagues for review.
- Improve the drafts based on the suggestions for improvement received from the reviewer colleagues.
- Finalize the SOPs, print them and put them in a new folder which you entitle "Equipment SOPs".
- Staff members who regularly use the pieces of equipment for which the SOPs have been written must read the SOPs. Include the SOP in the Read and Understand List and indicate which staff members have to read the SOP.
- Present the new SOPs to staff members in a weekly staff meeting and name the staff members who have to read the new SOPs. Explain that they must sign the Read and Understand List when they have read the SOP. Also explain where they can find the SOPs and the Read and Understand List.
- Regularly check that every staff member has read the SOPs. If some staff member lag too far behind, indicate this to the Laboratory Management.
Laboratory Manager:
- If the Quality Focal Point indicates that certain staff members lag too far behind in reading the SOPs assigned to them, motivate these staff members to read the SOPs.