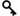
Title
Why
Each year, a lot of activities are undertaken in the laboratory. By making a Quality Policy that is based on a clear Vision, Mission, Core Values and Long Term Goals, these activities can all be pointed into one direction. This means that by planning the activities each year based on the Quality Policy:
- More relevant activities can be formulated
- Activities can be planned more efficiently
- The activities are better harmonized and working in synergy with the Quality Policy towards fulfilling the Long Term Goals.
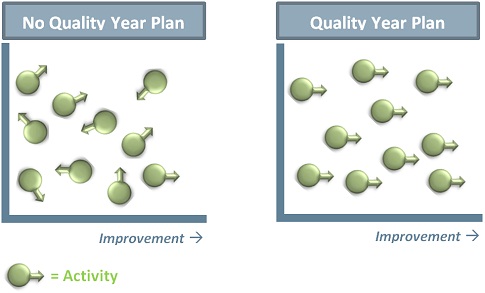
In one sentence: making a Quality Year Plan makes the laboratory move towards improvement instead of remaining in a stationary state (see figure).
In the right-hand column information is provided on organization planning and implementation from the WHO Laboratory Quality Management System (LQMS) handbook.
What
In the previous activity goals for the coming year were formulated. Make a plan describing the rationale behind each goal (explain: why was this goal formulated?) and how the goal should be achieved in this year (describe the approach). This is called the Quality Year Plan. Work closely together with the Quality Officer as he/she knows exactly how far the quality management system is implemented and what has yet to be done. They can provide an indication of what is achievable within one year and what is not.
The plan does not yet have to be written in SMART action points (this will be part of the next activity). However, the plans must be measurable: at the end of the year you must be able to check whether the plan has been completed. Therefore you must identify and describe an indicator for every activity described in the Quality Year Plan that helps you in monitoring progress in implementing the Quality Year Plan.
For the outline of the Quality Year Plan the Quality System Essentials (QSE) framework could be adapted. Be sure to include a table of contents, an introduction in which you summarize the Long Term Goals, and the Quality Policy , and provide a short description of the current situation. Explain the main focus of the Quality Year Plan of this year.
To get the best understanding of the contents, writing style and outline of a typical Quality Year Plan, see the Example Quality Year Plan added in the right-hand column (note that this example has another outline which does not follow the QSE framework). The example is a Quality Year Plan of a National Tuberculosis Reference Laboratory, however, the principle is the same for any other type of laboratory. The example Quality Year Plan also includes actions from a Management Review. For your first Quality Year Plan this will not be the case as you still have to do the first Management Review (which will be done later in this phase).
One important note: the Quality Year Plan does not necessarily have to run from 1 January until 31 December. It can also start and end in the middle of the calendar year, depending at what time of the year you do this activity. The date the Quality Year Plan is drafted should be taken as the start of the year. This is called the quality year.
How & who
Laboratory manager:
- Make a planning to achieve each goal for the coming year. Do this in discussion with the Quality Officer. Also identify indicators that the Quality Officer and you can use to monitor the implementation of the activities in the plan.
- Work this out into a complete Quality Year Plan.
- Take a new folder and name this "Management Reviews and Quality Year Plans". Insert two tabs entitled "Management Reviews" and "Quality Year Plans".
- Sign and date the Quality Year Plan and store this in the folder behind the correct tab.
- Document the procedure for making a Quality Year Plan in an SOP following the protocol for writing a Procedure SOP in the Master SOP and Using the template for a Procedure SOP attached to the Master SOP.
- Review and authorize the SOP for Making the Quality Year Plan according to the procedures described in the SOP for Document Control.
Quality Officer:
- Assist the Laboratory Manager where needed.