Title
Why
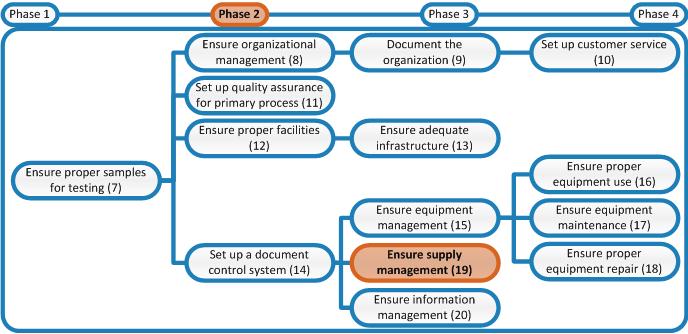
In this phase the Inventory Control System will be established. The first step is formulating the specifications of, and determining the number of supplies in stock. In the right-hand column general information on purchasing and inventory is provided from the WHO Laboratory Quality Management System handbook. [TB] You will also find here recommendations and tools more specific to TB laboratories.
What
The first step is to list all the supplies in the laboratory, determine which of those must always be in stock, and formulate their standard specifications. The standard specifications are requirements you have to formulate with regard to the characteristics you want the items to comply with. For example: a specification of the purity of a certain reagent, a specification of the minimum time to expiry, etc. Especially the minimum time to expiry is an important specification: it is a big problem that deliveries often take endlessly and then are almost expired upon arrival in limited resource countries.
How & who
Stock Officer:
- Make a list of items currently in stock. Do this using the Stock Cards.
- Determine whether there are any items missing.
- Determine which items must always be in stock and determine critical amounts (i.e. when new supply of this item needs to be ordered).
- Determine minimum and maximum amount for each item in stock based on use. Maximum amounts can help to prevent expiry of items, minimum amounts determine when to order a new supply of that item.
- Formulate the standard specifications of all supplies. These specifications are necessary for checking new deliveries when they arrive at the laboratory before placing them in the stock. You also need them for placing new orders to be sure that you order the correct product.
- List for each item the supplier details of the supplier normally used to order the item.
- The list you have now made will be used to set-up an inventory control system in the next activities.